



Valve-controlled sealLead acid batteryThere are two kinds: one is valve-controlled sealed lead-acid battery with ultra-fine glass fiber diaphragm (AGM), and the other is valve-controlled sealed lead-acid battery with colloid electrolyte (GFL) (abbreviated as GFL-VRLA battery). They all use the principle of cathodic absorption to seal the battery. Therefore, there must be a diaphragm gap of about 10% in the diaphragm of the AGM-VRLA battery. For the GFL-VRLA battery, after the perfused silica sol turns into a gel, the skeleton needs to further shrink, and the viscosity of the silica sol should be controlled around 10mPa.s, so that the gel cracks run through the positive and negative plates. Voids or cracks provide a passage to the negative electrode for oxygen released from the positive plate. In the production of AGM-VRLA battery, too much perfusion electrolyte is not conducive to the recombination of oxygen in the cathode, and filling too little electrolyte will increase the internal resistance of AGM-VRLA battery; in the production of GFL-VRLA battery, if the viscosity of silica sol is too high, that is, adding too much silicon solution, it will cause gel cracks and increase the internal resistance of GFL-VRLA battery, otherwise, it is not conducive to the recombination of oxygen in the cathode. Therefore, the production process of valve-controlled sealed lead-acid battery is very strict.
The colloidal electrolyte used in early GFL-VRLA batteries was made of water glass and then added directly to dry lead-acid batteries. Although this achieves the purpose of "fixing" the electrolyte or reducing the precipitation of acid fog, the capacity of the GFL-VRLA battery is about 20% lower than that of the ordinary lead-acid battery using the free electrolyte, so it is not accepted by people.
The research and development of GFL-VRLA battery was carried out in China in the 1950s. In the process of developing GFL-VRLA battery, the cathodic absorption battery with glass fiber diaphragm was born, which not only eliminates acid fog, but also shows the advantages of low internal resistance and good discharge characteristics at high current. Therefore, in the national economy, especially in the original use of ordinary lead-acid batteries, has been rapidly promoted and applied, during this period, the development of GFL-VRLA batteries in China is at a standstill.
In the 20th century, the GFL-VRLA battery product of Germany Sunshine Company entered the Chinese market, and its performance has been shown to be better than that of the early GFL-VRLA battery for many years. This makes the GFL-VRLA battery enter a new stage of development.
1. Major differences in structure and process
Whether it is AGM-VRLA battery or GFL-VRLA battery, they all use the principle of cathodic absorption to seal the battery. When the valve-controlled sealed lead-acid battery is charged, oxygen will be released from the positive electrode and hydrogen from the negative electrode. Positive oxygen evolution begins when the positive charge reaches 70%. The precipitated oxygen reaches the negative electrode and reacts with the negative electrode to achieve the purpose of cathodic absorption.
2Pb + O2=2PbO
2PbO + 2H2SO4:2PbS04+2H20
Hydrogen evolution from the negative electrode begins when the charge reaches 90%, coupled with the reduction of oxygen on the negative electrode and the increase of hydrogen overpotential of the negative electrode itself, thus avoiding a large number of hydrogen evolution reactions. For AGM-VRLA batteries, although most of the electrolyte of the battery is maintained in AGM-VRLA, 10% of the diaphragm pores must not enter the electrolyte, that is, lean design, through which the oxygen generated by the positive electrode reaches the negative electrode and is absorbed by the negative electrode.
For the GFL-VRLA battery, the GFL-VRLA battery is a three-dimensional porous network structure composed of SiO2 particles as the skeleton, which encapsulates the electrolyte inside. After the silica sol filled with GFL-VRLA battery becomes gel, the skeleton will further shrink, so that the gel cracks run through the positive and negative plates, providing a channel for the oxygen released from the positive electrode to reach the negative electrode.
It can be seen that the sealing principle of the two kinds of valve-controlled sealed lead-acid batteries is the same, and the difference lies in the "fixed" mode of the electrolyte and the way of providing oxygen to the negative electrode channel.
AGM-VRLA battery uses pure sulfuric acid aqueous solution as electrolyte and its density is 1.29~1.3lg/cm3. Except that part of the electrolyte is absorbed inside the plate, most of it exists in the glass fiber membrane. In order to make the electrode plate fully contact with the electrolyte, the pole group adopts the way of tight assembly. In addition, in order to ensure the sufficient life of the battery, the electrode plate should be designed to be thicker, and the positive grid alloy should adopt Pb-Ca-Sn-- A1 quaternary alloy.
The electrolyte of GFL-VRLA battery is composed of silica sol and sulfuric acid, and the concentration of sulfuric acid solution is lower than that of AGM-VRLA battery, usually 1.26~1.28g/cm3. The amount of electrolyte is 20% more than that of AGM-VRLA batteries, which is similar to that of ordinary lead-acid batteries. The electrolyte exists in a colloidal state and is filled in the diaphragm and between the positive and negative electrodes. The sulfuric acid electrolyte is surrounded by gel and does not flow out of the battery.
Because the GFL-VRLA battery adopts liquid-rich non-tight assembly structure, the positive grid material can be made of low antimony alloy or tubular positive plate. At the same time, in order to increase the capacity of GFL-VRLA battery without reducing the life of GFL-VRLA battery, the electrode plate can be made thinner. The internal space of the GFL-VRLA battery tank can also be expanded.
two。 Discharge capacity
The discharge capacity of the early GFL-VRLA battery is only about 80% of that of the ordinary lead-acid battery, which is due to the fact that the poor colloid electrolyte is directly injected into the ordinary lead-acid battery without modification, and the internal resistance of the GFL-VRLA battery is larger, which is caused by the difficulty of ion migration in the electrolyte. Recent research work shows that the formula of colloidal electrolyte is improved, the size of colloidal particles is controlled, hydrophilic polymer additives are added, the concentration of colloid is reduced and the permeability and affinity to the plate are increased. Vacuum filling process is adopted, and composite separator or AGM is used instead of rubber separator to improve the liquid absorption of GFL-VRLA battery. By canceling the sedimentation tank of GFL-VRLA battery and appropriately increasing the content of active material in the plate area, the discharge capacity of GFL-VRLA battery can reach or close to the level of ordinary lead-acid battery.
The amount of electrolyte of AGM-VRLA battery is less, the thickness of electrode plate is thicker, and the utilization rate of active material is lower than that of ordinary lead-acid battery, so the discharge capacity of AGM-VRLA battery is about 10% lower than that of ordinary lead-acid battery.
3. Internal resistance and high current discharge capacity
The glass fiber separator used in AGM-VRLA battery has 90% porosity, sulfuric acid is adsorbed inside, and AGM-VRLA battery is tightly assembled, so there is little hindrance to ion diffusion and electromigration in the separator, so AGM-VRLA battery has the characteristic of low internal resistance and strong ability of fast discharge with high current.
The electrolyte of GFL-VRLA battery is silica gel. Although the diffusion rate of ions in gel is close to that in aqueous solution, the migration and diffusion of ions are affected by the gel structure. The more curved the diffusion path of ions in the gel is, the narrower the pores in the structure are, the greater the hindrance is. Therefore, the internal resistance of GFL-VRLA battery is larger than that of AGM-VRLA battery.
However, the test results show that the high current discharge performance of GFL-VRLA battery is still very good, which fully meets the requirements of the relevant standards for high current discharge performance of storage battery. This is because the concentration of acid and other related ions in the liquid layer inside the porous electrode and near the plate plays a key role in high current discharge.
4. Thermal runaway
Thermal runaway means that there is a cumulative interaction between the charging current and temperature of the valve-controlled sealed lead-acid battery in the later stage of charging (or floating charge) because the charging voltage is not adjusted in time. At this time, the temperature of the valve-controlled sealed lead-acid battery rises sharply, which leads to the expansion and deformation of the valve-controlled sealed lead-acid battery tank and the increase of water loss rate. It even damages the valve-controlled sealed lead-acid battery.
The above phenomenon is a very destructive phenomenon when the AGM-VRLA battery is not used properly. This is due to the lean liquid tight assembly design of the AGM-VRLA battery, and 10% of the pores in the separator must be kept away from the electrolyte, so the internal thermal conductivity of the AGM-VRLA battery is poor and the thermal capacity is small. The oxygen produced by the positive electrode during charging will generate heat when it reaches the negative electrode and the negative lead react. if it is not conducted away in time, it will increase the temperature of the AGM-VRLA battery; if the charging voltage is not reduced in time, the charging current will increase and the oxygen evolution speed will increase, which in turn will increase the temperature of the AGM-VRLA battery. If such a vicious circle goes on, it will cause thermal runaway.
The amount of electrolyte of GFL-VRLA battery is equal to that of ordinary lead-acid battery, and the electrode group and the cell body are filled with gel electrolyte, which has large heat capacity and heat dissipation, and does not produce heat accumulation. Combined with the operation practice of GFL-VRLA battery for more than 30 years, no thermal runaway phenomenon of GFL-VRLA battery has been found.
5. service life
There are many factors affecting the service life of valve-controlled sealed lead-acid battery, including not only the design and manufacture of valve-controlled sealed lead-acid battery, but also the use and maintenance conditions of users. As far as the former is concerned, the corrosion resistance of positive grid and the water loss rate of valve-controlled sealed lead-acid battery are the two most important factors. Due to the increase of the thickness of the positive grid and the use of Pb-Ca-Sn-A1 quaternary corrosion resistant alloy, according to the corrosion rate of the grid, the service life of the valve-controlled sealed lead-acid battery can reach 10 to 15 years. However, from the application results of valve-controlled sealed lead-acid battery, the water loss rate has become the most key factor affecting the service life of valve-controlled sealed lead-acid battery.
Due to the lean liquid design of AGM-VRLA battery, the capacity of valve-controlled sealed lead-acid battery is very sensitive to the amount of electrolyte. Valve-regulated sealed lead-acid battery water loss of 10%, capacity will be reduced by 20%; loss of 25% water, AGM-VRLA battery life end. However, the GFL-VRLA battery adopts a liquid-rich design, and the electrolyte density is lower than that of the AGM-VRLA battery, which reduces the corrosion rate of grid alloy; the amount of electrolyte is also 15% to 20% more than that of the AGM-VRLA battery, and is less sensitive to water loss. All these measures are beneficial to prolong the service life of GFL-VRLA battery. According to the data provided by the German Sunshine Company, the colloidal electrolyte contains enough water to make the GFL-VRLA battery run for 12 to 14 years. In the first year of operation of the GFL-VRLA battery, the water loss was 4% to 5%, and then decreased year by year. After four years of operation, the annual water consumption was only 2%.
6. Compound efficiency
Recombination efficiency refers to the recombination ratio at which the oxygen produced by the positive electrode is absorbed by the negative electrode during charging. Factors such as charging current, temperature of valve-regulated sealed lead-acid battery, negative electrode characteristics and the speed of oxygen arriving at negative electrode all affect the gas recombination efficiency of valve-regulated sealed lead-acid battery.
According to the GFL-VRLA battery product manual provided by German Sunshine Company, the oxygen recombination efficiency of GFL-VRLA battery products is low in the early stage of use, but after several months of operation, the recombination efficiency can reach more than 95%. This phenomenon can also be verified by the water loss rate of the GFL-VRLA battery. In the first year of operation, the water loss rate of the GFL-VRLA battery was relatively large, reaching 4% to 5%, and gradually decreasing in the future. In the early stage of formation, the colloidal electrolyte has no or very few cracks inside, which does not provide enough channels for the oxygen precipitated from the positive electrode. As the colloid gradually shrinks, more and more channels will be formed, so the recombination efficiency of oxygen will gradually increase and water loss will inevitably decrease.
There are unsaturated voids in the AGM-VRLA battery separator, which provides a large number of oxygen channels, so its oxygen recombination efficiency is very high. The new AGM-VRLA battery can reach more than 98%.
7. conclusion
The performance comparison between GFL-VRLA batteries and AGM-VRLA batteries is shown in the following table.
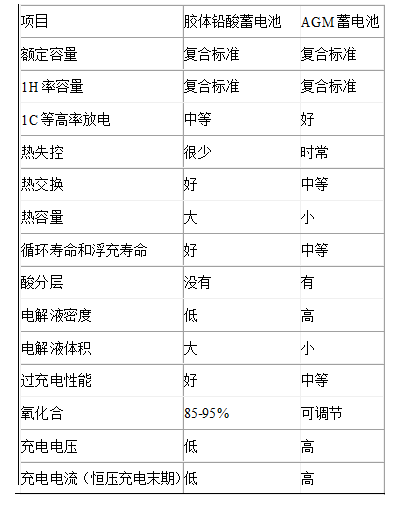
Performance comparison of GFL-VRLA battery and AGM-VRLA battery